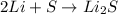The chemical equation given below represents the chemical reaction between lithium (li) and sulfur (s). in the equation, why is the number 2 present in front of lithium on the reactants side of the equation?
a. to show the number of lithium atoms involved in the reaction
b. to show the number of electrons gained by lithium in the reaction
c. to show the atomic number of lithium in the periodic table
d. to show the number of electrons lost by lithium in the reaction

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:00
What is the maximum number of electrons that an atomic orbital can contain?
Answers: 1

Chemistry, 22.06.2019 09:40
Which diagram shows the correct way to represent an ionic compound of magnesium oxide?
Answers: 3

Chemistry, 22.06.2019 16:30
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2

Chemistry, 22.06.2019 23:00
What is the name of the enzyme that forms at the start of transcription?
Answers: 1
You know the right answer?
The chemical equation given below represents the chemical reaction between lithium (li) and sulfur (...
Questions


Mathematics, 13.07.2019 08:00

Social Studies, 13.07.2019 08:00


Social Studies, 13.07.2019 08:00





Social Studies, 13.07.2019 08:00

Social Studies, 13.07.2019 08:00


History, 13.07.2019 08:00

Social Studies, 13.07.2019 08:00

History, 13.07.2019 08:00

History, 13.07.2019 08:00

History, 13.07.2019 08:00

History, 13.07.2019 08:00

History, 13.07.2019 08:00

History, 13.07.2019 08:00