
Chemistry, 09.09.2019 17:30 anitadefrances
Detailed calculations show that the value of z_eff for the outermost electrons m si and cl atoms is 4.29 + and 6.12 +, respectively. what value do you estimate for zeff experienced by the outermost electron in both si and cl by assuming core electrons contribute 1.00 and valence electrons contribute 0.00 to the screening constant?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
When curium-242 is bombarded with an alpha particle, two products are formed, one of which is a nudge on. what is the other product
Answers: 3

Chemistry, 22.06.2019 16:00
He table below gives the atomic mass and relative abundance values for the three isotopes of element m. relative abundance (%) atomic mass (amu) 78.99 23.9850 10.00 24.9858 11.01 25.9826 what is the average atomic mass (in amu) of element m? 2.86 5.36 24.30 24.98
Answers: 2

Chemistry, 22.06.2019 17:40
Which statement about hf is true? it is zero for any compound in its standard state. it is positive when the bonds of the product store more energy than those of the reactants. it is negative when a compound forms from elements in their standard states. it is zero for any element that is in the liquid state.
Answers: 1

Chemistry, 22.06.2019 18:30
Which of the following words describe the reality that the universe looks the same from various perspective
Answers: 3
You know the right answer?
Detailed calculations show that the value of z_eff for the outermost electrons m si and cl atoms is...
Questions




Mathematics, 13.12.2020 20:50

History, 13.12.2020 20:50

Biology, 13.12.2020 20:50

Biology, 13.12.2020 20:50

Mathematics, 13.12.2020 20:50


Mathematics, 13.12.2020 20:50

Mathematics, 13.12.2020 20:50


Chemistry, 13.12.2020 20:50





Chemistry, 13.12.2020 20:50


Computers and Technology, 13.12.2020 20:50

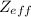 estimation for Si is 4, and for Cl it is 7.
estimation for Si is 4, and for Cl it is 7.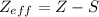 , with Z the atomic number of the element and the number of protons in the nucleus, and S the screening constant.
, with Z the atomic number of the element and the number of protons in the nucleus, and S the screening constant. 

