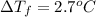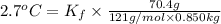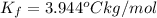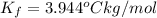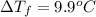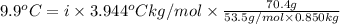Chemistry, 09.09.2019 18:30 chloejaylevesque
When 70.4 g of benzamide (c, h,no) are dissolved in 850. g of a certain mystery liquid x, the freezing point of the solution is 2.7 c lower than the freezing point of pure x. on the other hand, when 70.4 g of ammonium chloride (nh ci) are dissolved in the same mass of x, the freezing point of the solution is 9.9 °c lower than the freezing point of pure x. calculate the van't hoff factor for ammonium chloride in x. be sure your answer has a unit symbol, if necessary, and round your answer to 2 significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Electric charge is what ? a. kinetic energy b. radiation c. discovery d. electricity
Answers: 1

Chemistry, 22.06.2019 15:30
Why does earth rotate? because earth is formed from cold gases collapsing due to gravity because the matter in the nebula that formed earth was spinning because earth forms more than 99% of the mass of the solar system because the hydrogen atoms inside the nebula fused to form helium
Answers: 1

Chemistry, 22.06.2019 16:00
The chemical equation below shows the reaction of sodium (na) and chlorine (cl) to form sodium chloride (nacl). 2na + cl2 → 2nacl in this equation, which of the following is a reactant? i. sodium ii. chlorine iii. sodium chloride
Answers: 1

Chemistry, 22.06.2019 18:30
When the chemicals iron sulfide (fes) and hydrochloric acid (hcl) are combined, bubbles appear from the mixture. 1. does the appearance of bubbles indicate a physical or chemical change? 2. why do the bubbles indicate this change? 3. what property is this?
Answers: 1
You know the right answer?
When 70.4 g of benzamide (c, h,no) are dissolved in 850. g of a certain mystery liquid x, the freezi...
Questions

Biology, 14.04.2020 23:08



Social Studies, 14.04.2020 23:08

Chemistry, 14.04.2020 23:08


English, 14.04.2020 23:08

Mathematics, 14.04.2020 23:08

English, 14.04.2020 23:08

English, 14.04.2020 23:08




Mathematics, 14.04.2020 23:08


Mathematics, 14.04.2020 23:08


Mathematics, 14.04.2020 23:08


History, 14.04.2020 23:08

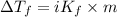
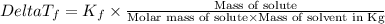 ...(1)
...(1)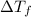 =Elevation in boiling point =
=Elevation in boiling point = 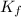 = Freezing point constant
= Freezing point constant