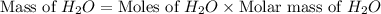Chemistry, 09.09.2019 19:30 Frenchfries13
Consider the following unbalanced chemical equation. c5h12(l) + o2(g) → co2(g) + h2o(l) if 21.9 grams of pentane (c5h12) are burned in excess oxygen, how many grams of h2o will be produced?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:00
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1


Chemistry, 22.06.2019 16:50
Which element is least likely to undergo a chemical reaction
Answers: 3

Chemistry, 23.06.2019 00:30
What is bromine+calcium iodide--> calcium bromide +iodine balanced
Answers: 1
You know the right answer?
Consider the following unbalanced chemical equation. c5h12(l) + o2(g) → co2(g) + h2o(l) if 21.9 gram...
Questions


History, 08.04.2020 20:38

Mathematics, 08.04.2020 20:38

History, 08.04.2020 20:39




Biology, 08.04.2020 20:39


Biology, 08.04.2020 20:39

Biology, 08.04.2020 20:39


English, 08.04.2020 20:39




Geography, 08.04.2020 20:39


Mathematics, 08.04.2020 20:39

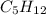 = 21.9 g
= 21.9 g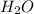 = 18 g/mole
= 18 g/mole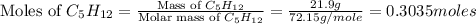
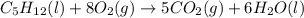
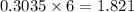 moles of
moles of