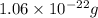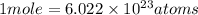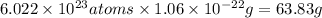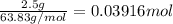Chemistry, 09.09.2019 21:30 ginocousins06
Acopper atom has a mass of 1 06 times 10^-22 g and a penny has a mass of 2.5 g. use this information to answer the questions below. be sure your answers have the correct number of significant digits. what is the mass of 1 mole of copper atoms? g how many moles of copper atoms have a mass equal to the mass of a penny?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:50
Suppose you got a low yield of benzoin from your benzoin condensation reaction and thus only have 0.300 g of benzoin to use as the starting material for this reaction. how much concentrated nitric acid should you add? (concentrated nitric acid is 15.8 m). write your answer in the form x.xx ml
Answers: 1

Chemistry, 22.06.2019 00:30
What does x represent in the formula for the compound xcl4?
Answers: 1

Chemistry, 22.06.2019 05:30
Which of the following two events occur to create a sea breeze? select all that apply. warm air rises on the ocean and moves toward the land to cool warm air rises on land and moves toward the ocean to cool cool air moves from the ocean to be warmed by the land cool air moves from the land to be warmed by the ocean
Answers: 3

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 3
You know the right answer?
Acopper atom has a mass of 1 06 times 10^-22 g and a penny has a mass of 2.5 g. use this information...
Questions

Mathematics, 28.02.2021 08:10

English, 28.02.2021 08:10

Mathematics, 28.02.2021 08:10

Computers and Technology, 28.02.2021 08:10

Social Studies, 28.02.2021 08:10


Mathematics, 28.02.2021 08:10



Mathematics, 28.02.2021 08:10

English, 28.02.2021 08:10

Mathematics, 28.02.2021 08:10



Mathematics, 28.02.2021 08:10

Mathematics, 28.02.2021 08:10

Mathematics, 28.02.2021 08:10