
Chemistry, 09.09.2019 23:30 webbhlharryteach
In the process of attempting to characterize a substance, a chemist makes the following observations. which are physical properties and which are chemical properties?
(a) the substance is a silvery white, lustrous metal
(b) it melts at 649°c and boils at 1105°c
(c) its density at 20°c is 1.738 g/cm3
(d) the substance burns in air, producing an intense

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Using the periodic table, complete the table to describe each atom. type in your answers
Answers: 3

Chemistry, 22.06.2019 16:50
Which element is least likely to undergo a chemical reaction
Answers: 3

Chemistry, 22.06.2019 17:30
A650 ml sodium bromine solution has a bromide ion concentration of 0.245 m. what is the mass (g) of sodium bromide in solution? a) 103.b)0.00155.c)16400.d) 16.4.e) 0.159
Answers: 2

Chemistry, 22.06.2019 18:00
How is energy related to the change of state represented by the model? atoms gain energy as a solid changes to a liquid. atoms gain energy as a solid changes to a gas. atoms lose energy as a solid changes to a liquid. atoms lose energy as a solid changes to a gas.
Answers: 3
You know the right answer?
In the process of attempting to characterize a substance, a chemist makes the following observations...
Questions

Chemistry, 07.05.2020 01:00







History, 07.05.2020 01:00


Mathematics, 07.05.2020 01:00

Mathematics, 07.05.2020 01:00




English, 07.05.2020 01:00

Mathematics, 07.05.2020 01:00


Mathematics, 07.05.2020 01:00

Mathematics, 07.05.2020 01:00

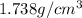 : physical property.
: physical property. 

