
Chemistry, 09.09.2019 23:30 jennypenny123
Which of the following statements are true concerning ionic bonding? select one: a. ionic bonding occurs between a metal, which has a high affinity for electrons, and a nonmetal, which loses electrons relatively easy. b. cacl2 forms because ca2+ is always a more stable species than the calcium atom alone. c. compounds with ionic bonds tend to have low melting points. d. the electronegativity difference between the bonding atoms of ionic compounds is small since the electrons are not shared but rather held together by electrostatic forces. e. all of the above statements are false.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:10
3.) for each of the following compounds, draw the major organic product of reaction with hcl or naoh and circle whether the starting materials and products will be more soluble in organic solvent or water benzoic acid + hcl: benzoic acid + naoh: oh benzoic acid water/organic water organic fluorenone hс: fluorenone + naoh: fluorenone water/organic water/organic веnzocaine + hci: benzocaine + n»oh: h2n benzocaine water/organic water organic o=
Answers: 3

Chemistry, 23.06.2019 04:31
How many grams of iron can be made from 16.5 grams of fe2o3
Answers: 1

Chemistry, 23.06.2019 05:50
Aseismic wave is energy released as the result of rock movement along a fault. t or f ?
Answers: 1

Chemistry, 23.06.2019 12:30
Question 1 (true/false worth 4 points) (03.06 lc) an induced dipole occurs when a molecule's moving electrons are briefly more concentrated in one place than another, causing the molecule to become temporarily polarized. true false
Answers: 2
You know the right answer?
Which of the following statements are true concerning ionic bonding? select one: a. ionic bonding...
Questions




History, 02.12.2019 01:31








Health, 02.12.2019 01:31






History, 02.12.2019 01:31


Mathematics, 02.12.2019 01:31

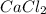 forms because
forms because 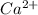 is always a more stable species than the calcium atom alone
is always a more stable species than the calcium atom alone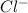 is an anion with oxidation state of -1. Thus they combine and their oxidation states are exchanged and written in simplest whole number ratios to give neutral
is an anion with oxidation state of -1. Thus they combine and their oxidation states are exchanged and written in simplest whole number ratios to give neutral ![[Ca]=1s^22s^22p^63s^23p^64s^2](/tpl/images/0226/1839/cdc07.png)
![[Ca^{2+}]=1s^22s^22p^63s^23p^6](/tpl/images/0226/1839/04411.png)
![[Cl]=1s^22s^22p^63s^23p^5](/tpl/images/0226/1839/7a3e4.png)
![[Cl^-]=1s^22s^22p^63s^23p^6](/tpl/images/0226/1839/bea8e.png)


