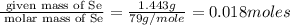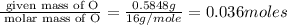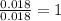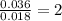Chemistry, 10.09.2019 00:30 chanceypray3514
Consider that a sample of a compound is decomposed and the masses of its constituent elements is as follows: 1.443 g se, 0.5848 g o what would be the empirical formula for this compound?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 18:30
When a device is used in a circuit in which the voltage is 81 v the current flowing through the device is 3 a what is the resistance of the device
Answers: 2

Chemistry, 22.06.2019 19:30
If 16.00g of hydrogen gas reacts with 126.73g of oxygen, how many grams of water are yielded? (both reactants are completely consumed in the reaction.)
Answers: 2

Chemistry, 22.06.2019 23:40
What energy conversion occurs when a sling shot is used to shoot a rock across the room? (2 points) question 2 options: 1) stored mechanical energy is converted to mechanical energy. 2) stored mechanical energy is converted to radiant energy. 3) gravitational energy is converted to radiant energy. 4) gravitational energy is converted to mechanical energy.
Answers: 1

Chemistry, 23.06.2019 00:30
An unknown insoluble substance displaced the water shown. it's mass is indicated on the triple beam balance. mass = a. 694 b. 693.5 c. 693.0 d.693.8
Answers: 1
You know the right answer?
Consider that a sample of a compound is decomposed and the masses of its constituent elements is as...
Questions

Chemistry, 29.09.2019 03:50

Computers and Technology, 29.09.2019 03:50

Biology, 29.09.2019 03:50

Chemistry, 29.09.2019 03:50


Mathematics, 29.09.2019 03:50



Biology, 29.09.2019 03:50

Mathematics, 29.09.2019 03:50


Social Studies, 29.09.2019 03:50


Mathematics, 29.09.2019 03:50



Mathematics, 29.09.2019 03:50

Physics, 29.09.2019 03:50

Chemistry, 29.09.2019 03:50

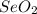 .
.