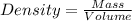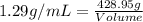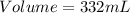The density of a solution of sulfuric acid is 1.29 g/cm3 and it is 38.1% acid by mass. what volume of the sulfuric acid solution is needed to supply 163 g of sulfuric acid? 1 cm3 = 1 ml 1. 428 g 2. 80.1 ml 3. 552 ml 4. 252 ml 5. 48.1 ml 6. 8010 ml 7. 332 ml 8. 0.00397 ml

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:30
What is the relation between concentration of reactants and the rate of chemical reaction?
Answers: 1

Chemistry, 22.06.2019 05:50
Significant figures are digits read directly from the measuring instrument plus one more digit, which is __ by the observer.
Answers: 2


Chemistry, 22.06.2019 14:50
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of tthe table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3
You know the right answer?
The density of a solution of sulfuric acid is 1.29 g/cm3 and it is 38.1% acid by mass. what volume o...
Questions


History, 04.08.2019 03:20





Mathematics, 04.08.2019 03:20

Mathematics, 04.08.2019 03:20

Mathematics, 04.08.2019 03:20

Biology, 04.08.2019 03:20

Mathematics, 04.08.2019 03:20








English, 04.08.2019 03:20

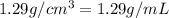
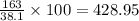 grams of solution of sulfuric acid
grams of solution of sulfuric acid