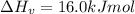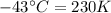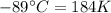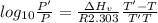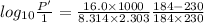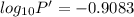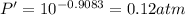Chemistry, 10.09.2019 01:30 natetheman7740
The enthalpy of vaporization of substance x is 16.0kj mol and its normal boiling point is −43.°c. calculate the vapor pressure of x at −89.°c. round your answer to 2 significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Describe the interaction that occurs between two objects with the same electrical charge.
Answers: 1

Chemistry, 22.06.2019 07:00
How heavy is thanos? a) 3000 lbs b) all of it c) the price of tea in china d) heavy enough
Answers: 2

Chemistry, 22.06.2019 07:30
In a reaction (at equilibrium) that makes more moles of gas than it consumes, what is the effect of increasing the pressure?
Answers: 1

Chemistry, 23.06.2019 02:00
Which of the following substances is the most soluble in water? a. sodium chloride b. methane c. bromine d. carbon
Answers: 1
You know the right answer?
The enthalpy of vaporization of substance x is 16.0kj mol and its normal boiling point is −43.°c. ca...
Questions


Mathematics, 26.02.2021 01:00

Mathematics, 26.02.2021 01:00





Chemistry, 26.02.2021 01:00

Mathematics, 26.02.2021 01:00

Mathematics, 26.02.2021 01:00


Mathematics, 26.02.2021 01:00


Mathematics, 26.02.2021 01:00






Mathematics, 26.02.2021 01:00

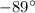 = 0.12 atm
= 0.12 atm