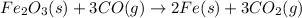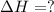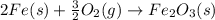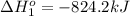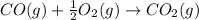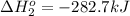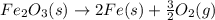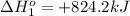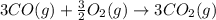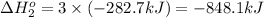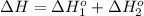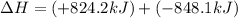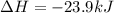Chemistry, 10.09.2019 02:30 lexibyrd120
Calculate δhrxn for the following reaction: fe₂o₃(s)+3co(g)→2fe(s)+3co₂(g) use the following reactions and given δh′s. 2fe(s)+3/2o₂(g)→fe₂o₃(s), δh = -824.2 kj co(g)+1/2o₂(g)→co₂(g), δh = -282.7 kj

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 19:10
Astudent completes a titration by adding 12.0 milliliters of naoh(aq) of unknown concentration to 16.0 milliliters of 0.15 m hcl(aq). what is the molar concentration of the naoh(aq)? 1)5.0 m 2)0.20 m 3)0.11 m 4)1.1 m
Answers: 1

Chemistry, 23.06.2019 03:30
Why do electrons further from the nucleus have more energy
Answers: 1

Chemistry, 23.06.2019 05:00
Which of the following describes qualitative data? a) recording the temperature of a solid as it is warmed. b) noting the color of a solution as it is heated. c) measuring the volume of an object by water displacement. d) taking the mass of an object using a balance.
Answers: 2

Chemistry, 23.06.2019 07:00
What is the difference between covalent bonds and ionic bonds? covalent bonds involve the sharing of electrons between atoms; ionic bonds involve the electrical attraction between charged atoms. covalent bonds involve the transfer of electrons between charged atoms; ionic bonds involve the sharing of electrons between atoms. covalent bonds involve the sharing of pairs of electrons between atoms; ionic bonds involve the sharing of single electrons between atoms. covalent bonds involve the sharing of electrons between atoms; ionic bonds involve the sharing of protons between charged atoms.
Answers: 1
You know the right answer?
Calculate δhrxn for the following reaction: fe₂o₃(s)+3co(g)→2fe(s)+3co₂(g) use the following reacti...
Questions




History, 01.04.2020 03:30


Mathematics, 01.04.2020 03:30


Mathematics, 01.04.2020 03:30


Mathematics, 01.04.2020 03:30



Social Studies, 01.04.2020 03:30


Mathematics, 01.04.2020 03:30

Biology, 01.04.2020 03:30

Mathematics, 01.04.2020 03:30

Chemistry, 01.04.2020 03:30

Social Studies, 01.04.2020 03:30

Mathematics, 01.04.2020 03:30