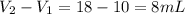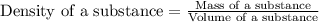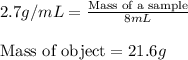Chemistry, 10.09.2019 13:10 Student3220
Asample of aluminum is placed in a 25 ml graduated cylinder containing 10 ml of water. the level of water rises to 18 ml. aluminum has a density of 2.7 g/ml. calculate the mass of the sample
include explanation if possible

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
What does x represent in the formula for the compound xcl4?
Answers: 1

Chemistry, 23.06.2019 02:00
Now look at the segment of the graph between the two data points marked with black squares. describe how the boiling point and melting point plots behave between these points. be as specific as possible.
Answers: 1

Chemistry, 23.06.2019 03:00
Determine type of reaction & predict the product c3h12+o2 =
Answers: 1

Chemistry, 23.06.2019 04:40
6) (a) calculate the absorbance of the solution if its concentration is 0.0278 m and its molar extinction coefficient is 35.9 l/(mol cm). the depth of the cell is 5 mm. (b) what is the %t? (7) calculate the absorbance of the solution if the transmitted light intensity is 70% of the initial light beam intensity
Answers: 1
You know the right answer?
Asample of aluminum is placed in a 25 ml graduated cylinder containing 10 ml of water. the level of...
Questions












Arts, 09.12.2019 19:31




Mathematics, 09.12.2019 19:31




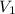 = 10 mL
= 10 mL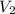 = 18 mL
= 18 mL