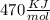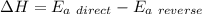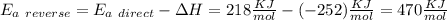The activation energy for the reaction no2 (g )+ co (g) ⟶ no (g) + co2 (g) is ea = 218 kj/mol and the change in enthalpy for the reaction is δh = -252 kj/mol . what is the activation energy for the reverse reaction? enter your answer numerically and in terms of kj/mol.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:00
In which of these cases are the two wave points considered to be in phase with each other?
Answers: 1

Chemistry, 22.06.2019 02:00
What is the maximum number of electrons that an atomic orbital can contain?
Answers: 1

Chemistry, 22.06.2019 12:00
Under normal conditions, describe how increasing the temperatures effects the solubility of a typical salt
Answers: 1

Chemistry, 22.06.2019 18:30
Which rate indicates the number of children that would be born per woman if she were to live to the end of her child bearing years
Answers: 2
You know the right answer?
The activation energy for the reaction no2 (g )+ co (g) ⟶ no (g) + co2 (g) is ea = 218 kj/mol and th...
Questions



Social Studies, 25.04.2020 04:51



English, 25.04.2020 04:51



Mathematics, 25.04.2020 04:51




Social Studies, 25.04.2020 04:51






Geography, 25.04.2020 04:51