
Chemistry, 10.09.2019 18:30 mikayleighb2019
Why does ammonium nitrate (nh4no3) dissolve readily in water even though the dissolution process is endothermic by 26.4 kj/mol? why does ammonium nitrate (nh4no3) dissolve readily in water even though the dissolution process is endothermic by 26.4 kj/mol? the vapor pressure of the water decreases upon addition of the solute. the osmotic properties of the system lead to this behavior. the overall enthalpy of the system decreases upon addition of the solute. the overall entropy of the system increases upon dissolution of this strong electrolyte. the overall enthalpy of the system increases upon dissolution of this strong electrolyte.

Answers: 1


Another question on Chemistry




Chemistry, 22.06.2019 18:30
Asample of hydrated tin (ii) chloride (sncl2) has a mass of 4.90 g. when it is dehydrated, it has a mass of 4.10 g. which is the correct chemical formula for the hydrate? sncl2•2h2o sncl2•4h2o sncl2•6h2o
Answers: 2
You know the right answer?
Why does ammonium nitrate (nh4no3) dissolve readily in water even though the dissolution process is...
Questions

Social Studies, 08.11.2019 04:31




Business, 08.11.2019 04:31




Mathematics, 08.11.2019 04:31


English, 08.11.2019 04:31

Mathematics, 08.11.2019 04:31





Mathematics, 08.11.2019 04:31

Mathematics, 08.11.2019 04:31

Mathematics, 08.11.2019 04:31

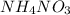 is ionic in nature. Hence, when it is added to water then it will readily dissociate into ammonium ions (
is ionic in nature. Hence, when it is added to water then it will readily dissociate into ammonium ions (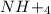 ) and nitrate ions (
) and nitrate ions (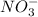 ).
).

