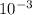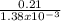The partial pressure of o2 in air at sea level is 0.21atm. the solubility of o2 in water at 20∘c, with 1 atm o2 pressure is 1.38×10−3 m. part a using henry's law, calculate the molar concentration of o2 in the surface water of a mountain lake saturated with air at 20 ∘c and an atmospheric pressure of 665 torr . express your answer using two significant figures. nothing

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:00
If a plot weight (in g) vs. volume (in ml) for a metal gave the equation y= 13.41x and r^2=0.9981 what is the density of the metal?
Answers: 2

Chemistry, 22.06.2019 16:50
Which of the following is an indication that a substance has undergone a chemical change? a. no new product has been formed. b. the color of the substance has not changed. c. the original constitute has not changed. d. the molecular structure has changed.
Answers: 1

Chemistry, 22.06.2019 19:30
Awoman's basketball has a circumference between 28.5 and 29.0 inches and a maximum weight of 20 ounces (two significant figures). what are these specifications in units of centimeters and grams?
Answers: 2

Chemistry, 23.06.2019 01:00
Reactions in cells take place at about a. 40°c b. 0° c. 100°c d. 60°c
Answers: 1
You know the right answer?
The partial pressure of o2 in air at sea level is 0.21atm. the solubility of o2 in water at 20∘c, wi...
Questions

Advanced Placement (AP), 22.08.2019 16:20

Mathematics, 22.08.2019 16:20


Advanced Placement (AP), 22.08.2019 16:20

Mathematics, 22.08.2019 16:20


Mathematics, 22.08.2019 16:20









Computers and Technology, 22.08.2019 16:20




Mathematics, 22.08.2019 16:20

 M
M