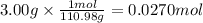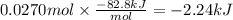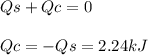Chemistry, 10.09.2019 19:30 santos200154
In the following experiment, a coffee-cup calorimeter containing 100 ml of h2o is used. the initial temperature of the calorimeter is 23.0 ∘c. if 3.00 g of cacl2 is added to the calorimeter, what will be the final temperature of the solution in the calorimeter? the heat of solution δhsoln of cacl2 is −82.8 kj/mol. assume that the specific heat of the solution form

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:00
How many liters of water vapor can be produced if 108 grams of methane gas (ch4) are combusted at 312 k and 0.98 atm? show all work. pls ! will mark as brainliest
Answers: 1

Chemistry, 22.06.2019 00:40
During which time interval does the object travel approximately 10 meters
Answers: 3

Chemistry, 22.06.2019 08:30
In the reaction between a crushed antacid tablet and vinegar what gas is emitted
Answers: 2

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 2
You know the right answer?
In the following experiment, a coffee-cup calorimeter containing 100 ml of h2o is used. the initial...
Questions



Mathematics, 30.01.2020 12:58






Mathematics, 30.01.2020 12:58


History, 30.01.2020 12:58


Mathematics, 30.01.2020 12:58


English, 30.01.2020 12:58

Social Studies, 30.01.2020 12:58