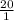Chemistry, 10.09.2019 20:30 dreyiamacc9695
The ph of blood depends on the [hco3-/h2co3] balance. ([h2co3] is equal to the amount of dissolved co2). calculate the bicarbonate (hco3-) : carbon dioxide ratio for a normal blood ph of 7.40. (the pka1 of carbonic acid is 6.10 at 37oc, body temperature). (a) 20 : 1 (b) 1.3 : 1 (c) 2 : 1 (d) 1 : 20 (e) 1 : 0.01

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:00
The human eye contains a molecule called 11-cis-retinal that changes shape when struck with light of sufficient energy. the change in shape triggers a series of events that results in an electrical signal being sent to the brain that results in vision. the minimum energy required to change the conformation of 11-cis-retinal within the eye is about 164 kj/mol.
Answers: 2

Chemistry, 22.06.2019 12:00
Which statement best explains the relationship between an area is geography and the temperature of its surface water
Answers: 1

Chemistry, 23.06.2019 13:00
How many grams of oxygen gas will react completely with a block of calcium metal that is 3.0 cm by 3.5 cm by 4.2 cm, if the density of calcium is 1.55 g/ml? show all steps of your calculation as well as the final answer.
Answers: 3

Chemistry, 23.06.2019 16:10
Which is the best term to use when describing the energy of position? chemical kinetic potential electromagnetic
Answers: 3
You know the right answer?
The ph of blood depends on the [hco3-/h2co3] balance. ([h2co3] is equal to the amount of dissolved c...
Questions


Geography, 09.02.2021 19:10


Mathematics, 09.02.2021 19:10


Mathematics, 09.02.2021 19:10


Mathematics, 09.02.2021 19:10

Arts, 09.02.2021 19:10

Social Studies, 09.02.2021 19:10



Geography, 09.02.2021 19:10


Advanced Placement (AP), 09.02.2021 19:10

Mathematics, 09.02.2021 19:10




Mathematics, 09.02.2021 19:10

![[H_{2}CO_{3}]](/tpl/images/0226/9901/77e8d.png) =
= ![[CO_{2}]](/tpl/images/0226/9901/0a7e9.png)
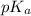 = 6.10
= 6.10![\frac{[HCO_^{-}{3}]}{[CO_{2}]}](/tpl/images/0226/9901/0b823.png) = ?
= ?![pK_{a} + log_{10} \frac{[Salt]}{[Acid]}](/tpl/images/0226/9901/394fa.png)
![pK_{a} + log_{10} \frac{[HCO^{-}_{3}]}{[H_{2}CO_{3}]}](/tpl/images/0226/9901/d9b18.png)
![log_{10} \frac{[HCO^{-}_{3}]}{[H_{2}CO_{3}]}](/tpl/images/0226/9901/e29e6.png)
![\frac{[HCO^{-}_{3}]}{[H_{2}CO_{3}]}](/tpl/images/0226/9901/4d8f1.png) = antilog (1.30)
= antilog (1.30) ![\frac{[HCO^{-}_{3}]}{[CO_{2}]}](/tpl/images/0226/9901/7a396.png) =
=