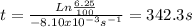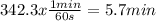Chemistry, 10.09.2019 22:30 liltinyhead
Acertain first-order reaction (a→products) has a rate constant of 8.10×10−3 s−1 at 45 ∘c. how many minutes does it take for the concentration of the reactant, [a], to drop to 6.25% of the original concentration? express your answer with the appropriate units.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Calculate the h3o+ concentration in a solution of acetic acid if the concentration of molecular acetic acid present at equilibrium is 9.97x10^-3 m and k for the dissociation is 1.86x10^-5. ch3cooh(aq)+h2o(> h3o^+(aq)+ch3coo^-(aq)
Answers: 2


Chemistry, 22.06.2019 15:20
Draw any one of the skeletal structures of a 2° alkyl bromide having the molecular formula of c6h13br and two stereogenic centers. indicate chirality by using wedge and hashed wedge notation. lone pairs do not need to be shown.
Answers: 1

Chemistry, 22.06.2019 22:30
Why is it possible for different microorganisms to extract energy not only from carbohydrates and other biological molecules but from a large variety of substances?
Answers: 1
You know the right answer?
Acertain first-order reaction (a→products) has a rate constant of 8.10×10−3 s−1 at 45 ∘c. how many m...
Questions

English, 16.10.2019 10:30




Mathematics, 16.10.2019 10:30


Mathematics, 16.10.2019 10:30

Physics, 16.10.2019 10:30

Physics, 16.10.2019 10:30






Business, 16.10.2019 10:30



Mathematics, 16.10.2019 10:30


Mathematics, 16.10.2019 10:30

![Ln [A] = -k.t + Ln [A]_{0}](/tpl/images/0227/1833/30b90.png) . Where [A] is the concentration of the reactant at any t time of the reaction,
. Where [A] is the concentration of the reactant at any t time of the reaction, ![[A]_{0}](/tpl/images/0227/1833/48818.png) is the concentration of the reactant at the beginning of the reaction and k is the rate constant.
is the concentration of the reactant at the beginning of the reaction and k is the rate constant. ![[A]=\frac{6.25}{100}.[A]_{0}](/tpl/images/0227/1833/4f27b.png) . And the rate constant (k) is 8.10×10−3 s−1
. And the rate constant (k) is 8.10×10−3 s−1![Ln \frac{6.25}{100}.[A]_{0} = -8.10x10^{-3}s^{-1}.t + Ln[A]_{0}](/tpl/images/0227/1833/587cc.png)
![Ln [A]_{0}.\frac{6.25}{100} - Ln [A]_{0} = -8.10x10^{-3}s^{-1}.t](/tpl/images/0227/1833/49854.png)
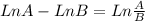
![Ln \frac{[A]_{0}}{[A]_{0}}.\frac{6.25}{100} = -8.10x10^{-3}s^{-1}.t](/tpl/images/0227/1833/72778.png)