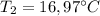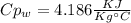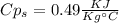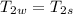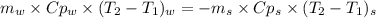Chemistry, 11.09.2019 00:30 sedratkawaiah13
A1.28-kg sample of water at 10.0 °c is in a calorimeter. you drop a piece of steel with a mass of 0.385 kg at 215 °c into it. after the sizzling subsides, what is the final equilibrium temperature? (make the reasonable assumptions that any steam produced condenses into liquid water during the process of equilibration and that the evaporation and condensation don’t affect the outcome, as we’ll see in the next section.)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:20
Much of the general structure and physical properties of the interior of the earth are inferred from: a)deep oil and gas bore holes b)geologic investigations c)analysis of seismic waves d) study of volcanoes
Answers: 1

Chemistry, 22.06.2019 13:30
Ants live on acacia trees in south america. the ants feed on sugars secreted by the trees. the trees provide room for the ants to live. the ants sting any other insect or animal that comes to eat the trees. what type of relationship is this?
Answers: 1


Chemistry, 22.06.2019 23:00
What is formed when amino acids form long chains or polymerize
Answers: 1
You know the right answer?
A1.28-kg sample of water at 10.0 °c is in a calorimeter. you drop a piece of steel with a mass of 0....
Questions


Mathematics, 22.10.2020 22:01

English, 22.10.2020 22:01

Advanced Placement (AP), 22.10.2020 22:01


Mathematics, 22.10.2020 22:01

Mathematics, 22.10.2020 22:01


History, 22.10.2020 22:01

History, 22.10.2020 22:01

English, 22.10.2020 22:01


Mathematics, 22.10.2020 22:01



Mathematics, 22.10.2020 22:01

Mathematics, 22.10.2020 22:01


Engineering, 22.10.2020 22:01

History, 22.10.2020 22:01