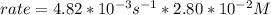Chemistry, 11.09.2019 00:30 nicholasryanencarnac
In carbon tetrachloride proceeds as follows: 2n2o5→4no2+o2. the rate law is first order in n2o5. at 64 ∘c the rate constant is 4.82 ×10−3s−1. part a part complete write the rate law for the reaction. write the rate law for the reaction. rate=2.41×10−3s−1[n2o5] rate=4.82×10−3s−1[n2o5] rate=9.64×10−3s−1[n2o5] rate=4.82×10−3s−1[n2o5]2 previous answers correct part b what is the rate of reaction when [n2o5]= 2.80×10−2 m ?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 16:30
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3


Chemistry, 22.06.2019 21:20
40dm3 of gas at 760 torr are heated from 5°c to 50°c what is the new volume
Answers: 3

You know the right answer?
In carbon tetrachloride proceeds as follows: 2n2o5→4no2+o2. the rate law is first order in n2o5. at...
Questions

Mathematics, 17.05.2021 22:30

Geography, 17.05.2021 22:30

History, 17.05.2021 22:30

Mathematics, 17.05.2021 22:30

Chemistry, 17.05.2021 22:30

Computers and Technology, 17.05.2021 22:30


Social Studies, 17.05.2021 22:30


Mathematics, 17.05.2021 22:30

Mathematics, 17.05.2021 22:30



English, 17.05.2021 22:30

Mathematics, 17.05.2021 22:30


History, 17.05.2021 22:30


Mathematics, 17.05.2021 22:30

Mathematics, 17.05.2021 22:30

![rate= 4.82*10^{-3}s^{-1} * [N2O5]](/tpl/images/0227/3013/f16e4.png)
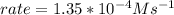
![rate= k * [N2O5]^{x}](/tpl/images/0227/3013/f017a.png)