
Chemistry, 11.09.2019 03:10 cmflores3245
Asample of pure water is heated to a temperature of 112 c at a pressure of 20 mpa, where the ionization constant for water is 4.0 x 1012. what is the oh concentration in the pure water at these conditions?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:00
What is the lowest number energy level where a d sublevel is found
Answers: 1


Chemistry, 22.06.2019 14:30
How can carbon move from "land" to bodies of water? describe the way human impact has lead to increased levels of co2 in the atmosphere.
Answers: 2

Chemistry, 22.06.2019 20:00
There are two steps in the usual industrial preparation of acrylic acid, the immediate precursor of several useful plastics. in the first step, calcium carbide and water react to form acetylene and calcium hydroxide: cac2 (s) + 2h2o (g) → c2h2 (g) + caoh2 (s) =δh−414.kj in the second step, acetylene, carbon dioxide and water react to form acrylic acid: 6c2h2 (g) + 3co2 (g) + 4h2o (g) → 5ch2chco2h (g) =δh132.kj calculate the net change in enthalpy for the formation of one mole of acrylic acid from calcium carbide, water and carbon dioxide from these reactions. round your answer to the nearest kj .
Answers: 3
You know the right answer?
Asample of pure water is heated to a temperature of 112 c at a pressure of 20 mpa, where the ionizat...
Questions

History, 09.03.2021 03:00

Mathematics, 09.03.2021 03:00


Mathematics, 09.03.2021 03:00



Mathematics, 09.03.2021 03:00


Mathematics, 09.03.2021 03:00


Biology, 09.03.2021 03:00

Physics, 09.03.2021 03:00

Biology, 09.03.2021 03:00



Mathematics, 09.03.2021 03:00

Mathematics, 09.03.2021 03:00

Chemistry, 09.03.2021 03:00


Mathematics, 09.03.2021 03:00

![[OH^-]^2=2.0\times 10^{6}](/tpl/images/0227/4886/54276.png)
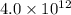 at a temperature of
at a temperature of 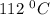 and pressure of 20 MPa
and pressure of 20 MPa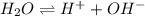
![K_w=[H^+][OH^-]](/tpl/images/0227/4886/bc68a.png)
![[H^+]=[OH^-]](/tpl/images/0227/4886/0669d.png)
![K_w=[OH^-]^2](/tpl/images/0227/4886/d08d4.png)
![[OH^-]^2=4.0\times 10^{12}](/tpl/images/0227/4886/f96fa.png)


