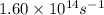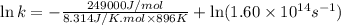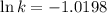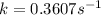Chemistry, 11.09.2019 04:30 shawn20034
Ethyl chloride vapor decomposes by the first-order reaction c2h5cl → c2h4 + hcl the activation energy is 249 kj/mol and the frequency factor is 1.60 × 1014 s−1. find the value of the specific rate constant at 896 k . enter your answer numerically (to 4 decimal places) and in terms of the appropriate units for a first order reaction.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:40
Write the formula for the following chemicals. 7. e. trinitrogen tetraoxide a calcium phosphate f. magnesium acetate b. potassium sulfide g nickel(iii) cyanide c carbon dioxide h. silver sulfate d. cobalt(ii) chloride
Answers: 1

Chemistry, 22.06.2019 10:00
Select all of the methods through which a drug can enter your body. injection swallowing inhalation absorption
Answers: 2

Chemistry, 22.06.2019 19:40
Scientists have developed an explanation of a phenomenon from several verified hypotheses. the explanation has been confirmed through numerous experimental tests.which option best describes this explanation? a. scientific lawb. research questionc. hypothesisd. scientific theory
Answers: 3

Chemistry, 23.06.2019 04:20
Malia was able to make a paper clip float on the surface of water what will most likely happen to the paper clip if a drop of dishwashing detergent is added near it
Answers: 1
You know the right answer?
Ethyl chloride vapor decomposes by the first-order reaction c2h5cl → c2h4 + hcl the activation energ...
Questions



History, 19.01.2021 23:30

Mathematics, 19.01.2021 23:30

Health, 19.01.2021 23:30


Mathematics, 19.01.2021 23:30

English, 19.01.2021 23:30




History, 19.01.2021 23:30








Mathematics, 19.01.2021 23:30

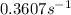
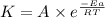
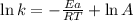 ............(1)
............(1)