
Chemistry, 11.09.2019 04:30 revlonknox6
The following substances dissolve when added to water. classify the substances according to the strongest solute-solvent interaction that will occur between the given substances and water during dissolution.
(1) ion-ion forces
(2) dipole dipole forces
(3) ion dipole forces
(4) london dispersion forces
(a) hf
(b) ch3oh
(c) cacl2
(d) febr3

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:00
When the following equation is balanced using the smallest possible integers, what is the coefficent of oxygen gas? c7h16o(g) + o2(g) → co2(g) + h2o(g) -1 -5 -8 -16 -21
Answers: 3


Chemistry, 22.06.2019 07:30
All cells are made of four types of acids, lipids, proteins, and carbohydrates.
Answers: 1

Chemistry, 22.06.2019 13:50
How does the motion of particles in a gas change as the gas cools
Answers: 2
You know the right answer?
The following substances dissolve when added to water. classify the substances according to the stro...
Questions


Mathematics, 23.01.2021 22:10

Mathematics, 23.01.2021 22:10

Mathematics, 23.01.2021 22:10


Mathematics, 23.01.2021 22:10









Biology, 23.01.2021 22:10


Mathematics, 23.01.2021 22:10

Physics, 23.01.2021 22:10


English, 23.01.2021 22:10

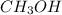 - There will also be development of partial charges due to the difference in electronegativity of oxygen and hydrogen atoms.
- There will also be development of partial charges due to the difference in electronegativity of oxygen and hydrogen atoms. 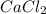 - It is an ionic compound. Hence, there will be partial positive charge on calcium and partial negative charge on chlorine atom.
- It is an ionic compound. Hence, there will be partial positive charge on calcium and partial negative charge on chlorine atom.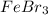 - It is an ionic compound. Hence, there will also exist ion-ion forces.
- It is an ionic compound. Hence, there will also exist ion-ion forces.

