
Chemistry, 12.09.2019 01:10 maehardy4134
Solutions of sodium carbonate and silver nitrate react to form solid silver carbonate and a solution of sodium nitrate. a solution containing 3.60g of sodium carbonate is mixed with one containing 5.14 of silver nitrate.
1.) how many grams of sodium carbonate are present after the reaction is complete?
2.)how many grams of silver nitrate are present after the reaction is complete?
3.)how many grams of silver carbonate are present after the reaction is complete?
4.) how many grams of sodium nitrate are present after the reaction is complete?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Identify the missing numbers below to show the result of multiplying the numbers (1.6 × 10-19)(5.0 × 106) = c × 10d
Answers: 1

Chemistry, 22.06.2019 14:30
Select all of the statements which are true. electrons are located in shells or orbits around the atom. electrons orbit slowly around the atom. electrons travel in one flat path around the nucleus of an atom. the valence of an atom is determined by the number of electrons in the atom's outermost shell.
Answers: 1

Chemistry, 22.06.2019 20:00
Which of the following would not diffuse through the plasma membrane by means of simple diffusion? 1 oxygen 2 glucose 3 a steroid hormone 4 a lipid soluble vitamin
Answers: 3

Chemistry, 22.06.2019 21:00
What type of radiation is lead emitting in the following equation? alpha particles beta particles gamma rays
Answers: 3
You know the right answer?
Solutions of sodium carbonate and silver nitrate react to form solid silver carbonate and a solution...
Questions

Chemistry, 21.10.2020 02:01


Spanish, 21.10.2020 02:01

Chemistry, 21.10.2020 02:01

Mathematics, 21.10.2020 02:01



Social Studies, 21.10.2020 02:01

Chemistry, 21.10.2020 02:01





History, 21.10.2020 02:01

English, 21.10.2020 02:01

Social Studies, 21.10.2020 02:01




Arts, 21.10.2020 02:01

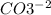 , and the ion nitrate is
, and the ion nitrate is 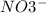 .
. 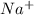 . Silver has only one electron too, so the cation will be
. Silver has only one electron too, so the cation will be 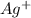 .
. 

