
Chromium has an atomic mass of 51.9961 u and consists of four isotopes, cr50, cr52, cr53, and cr54. the cr52 isotope has a natural abundance of 83.79% and an atomic mass of 51.9405 u. the cr54 isotope has a natural abundance of 2.37% and an atomic mass of 53.9389 u. the natural abundances of the cr50 and cr53 isotopes exist in a ratio of 1: 0.4579, and the cr50 isotope has an atomic mass of 49.9460 u. determine the atomic mass of the cr53 isotope.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Water's surface tension and heat storage capacity are accounted for by its a) orbitals b) weight c) hydrogen bonds d) mass e) size
Answers: 2

Chemistry, 22.06.2019 16:40
Let the ed50 of a recreational drug be defined as the amount required for 50% of a test group to feel high or get a buzz. if the ed50 value of ethanol is 470 mg/kg body mass, what dose would a 70 kg party goer need to quickly consume in order to have a 50% chance of getting a buzz? 235 mg 470 mg 32,900 mg 35,000,000 mg
Answers: 3

Chemistry, 22.06.2019 20:30
Water undergoes a large change in density at 0 ∘ c as it freezes to form ice. calculate the percent change in density that occurs when liquid water freezes to ice at 0 ∘ c given that
Answers: 2

Chemistry, 23.06.2019 00:50
Which of the following warnings would an agricultural chemist tell a farmer who wants to recycle his or her own ammonia? recycling ammonia is a difficult process that sometimes takes weeks. recycling ammonia requires a degree in biochemistry or a related field. recycling ammonia can be harmful because it is highly flammable and toxic. recycling ammonia costs too much money considering the price of the necessary chemicals.
Answers: 1
You know the right answer?
Chromium has an atomic mass of 51.9961 u and consists of four isotopes, cr50, cr52, cr53, and cr54....
Questions


English, 23.11.2020 20:30

Mathematics, 23.11.2020 20:30




English, 23.11.2020 20:30





Physics, 23.11.2020 20:30

History, 23.11.2020 20:30

English, 23.11.2020 20:30


English, 23.11.2020 20:30




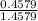 x 13.84 = 4.3469%
x 13.84 = 4.3469%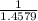 x 13.84u = 9.4931%
x 13.84u = 9.4931%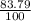 ) + ( 49.9460 x
) + ( 49.9460 x 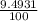 + (m₅₃ x
+ (m₅₃ x 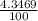 ) + (53.9389 x
) + (53.9389 x 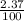 )
)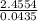 = 56.4459u
= 56.4459u

