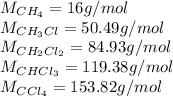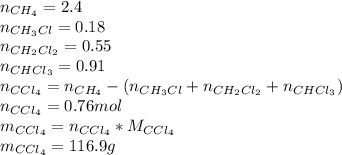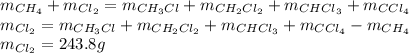Chemistry, 12.09.2019 19:20 tishfaco5000
Methane and chlorine react to form four products: ch3cl, ch2cl2, chcl3, and ccl4. at a particular temperature and pressure, 38.4 g of ch4 was allowed to react with excess cl2 and gave 9.2 g ch3cl, 47.1 g ch2cl2, and 109 g chcl3. all the ch4 reacted. (note: the hydrogen that is displaced from the carbon also combines with cl2 to form hcl.)how many grams of ccl4 were formed? how many grams of cl2 reacted with the ch4?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:30
The table describes how some substances were formed substance 19 description formed by boiling pure water formed by combining three hydrogen atoms to every nitrogen atom formed by adding 5 g of sugar to 1 l of water formed by compressing carbon under high pressure based on the given descriptions, which substance is most likely a mixture?
Answers: 1

Chemistry, 22.06.2019 12:40
When 13.3 g koh is dissolved in 102.7 g of water in a coffee-cup calorimeter, the temperature rises from 21.4 °c to 31.53 °c. what is the enthalpy change per gram of koh (j/g) dissolved in the water? * take the density of water as 1.00 g/ml. * assume that the solution has a specific heat capacity of 4.18 j/g*k. enter to 1 decimal place. do not forget the appropriate sign /(+). canvas may auto-delete the (+) sign
Answers: 2

Chemistry, 22.06.2019 19:30
Which one of the following substances would be the most soluble in ccl4? na2so4 h2o ch3ch2ch2ch2oh c4h10 hi
Answers: 1

Chemistry, 23.06.2019 00:00
How many peaks will be present in a mass spectrum for brcl?
Answers: 1
You know the right answer?
Methane and chlorine react to form four products: ch3cl, ch2cl2, chcl3, and ccl4. at a particular t...
Questions


History, 28.08.2019 23:30



English, 28.08.2019 23:30


History, 28.08.2019 23:30

Mathematics, 28.08.2019 23:30

Biology, 28.08.2019 23:30

Geography, 28.08.2019 23:30

Chemistry, 28.08.2019 23:30

Biology, 28.08.2019 23:30

History, 28.08.2019 23:30




Mathematics, 28.08.2019 23:30

Mathematics, 28.08.2019 23:30

History, 28.08.2019 23:30

Geography, 28.08.2019 23:30