Chemistry, 12.09.2019 20:10 ayoismeisjjjjuan
For the reaction a+b+c=> d+e, the initial reaction rate was measured for various initial concentrations of reactants. the following data were collected:
trial a(m) ( c(m) initial rate(m/s)
1 0.40 0.40 0.40 1.2 x 10^-4
2 .40 0.40 .20 .6 x 10^-4
0.80 . 0.40 4.8 x 10^-4
4 0.80 .80 .40 .8 x 10^-4
what is the value of the rate constant k for this reaction?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:30
What is i fracture in the crust called when land move up, down or sideways
Answers: 2


Chemistry, 23.06.2019 00:00
If many scientists conduct the same or similar experiments, and all obtain similar results, a can be written, which is a generally agreed-upon statement that explains and predicts how a natural phenomenon works.
Answers: 1

Chemistry, 23.06.2019 03:00
Describe the properties of sodium, chlorine, and sodium chloride
Answers: 1
You know the right answer?
For the reaction a+b+c=> d+e, the initial reaction rate was measured for various initial concentr...
Questions



Biology, 25.09.2019 18:50




Mathematics, 25.09.2019 18:50


Geography, 25.09.2019 18:50


Business, 25.09.2019 19:00


Mathematics, 25.09.2019 19:00

Social Studies, 25.09.2019 19:00


Chemistry, 25.09.2019 19:00

Computers and Technology, 25.09.2019 19:00

English, 25.09.2019 19:00


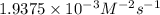
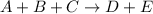
![\text{Rate}=k[A]^a[B]^b[C]^c](/tpl/images/0229/0682/be89a.png)
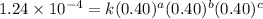 ....(1)
....(1)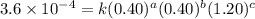 ....(2)
....(2)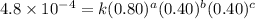 ....(3)
....(3)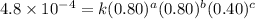 ....(4)
....(4)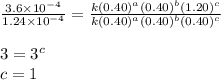
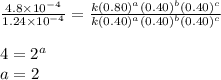
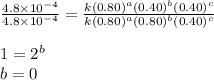
![\text{Rate}=k[A]^2[B]^0[C]^1](/tpl/images/0229/0682/54afd.png)