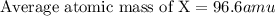An imaginary element (x) on mars is composed of three isotopes, 10.68% of isotope x-95 with a mass of 95.0 amu, 16.90% of isotope x-96 with a mass of 96.0 amu, and 72.42% of isotope x-97 with a mass of 97.0 amu. calculate the atomic mass (in amu) of the element. type in your answer with 3 significant figures.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Calculate the ratio of h+ ions to oh– ions at a ph = 7. find the concentration of h+ ions to oh– ions listed in table b of your student guide. then divide the h+ concentration by the oh– concentration. record this calculated ratio in table a of your student guide. compare your approximated and calculated ratios of h+ ions to oh– ions at a ph = 7. are they the same? why or why not? record your comparison in table a. what is the concentration of h+ ions at a ph = 7? mol/l what is the concentration of oh– ions at a ph = 7? mol/l what is the ratio of h+ ions to oh– ions at a ph = 7? : 1
Answers: 1



Chemistry, 22.06.2019 22:30
Which statement best summarizes the importance of ernest rutherford’s gold foil experiment? it proved that all of john dalton’s postulates were true. it verified j. j. thomson’s work on the atomic structure. it showed that an electron circles a nucleus in a fixed-energy orbit. it showed that a nucleus occupies a small part of the whole atom.
Answers: 1
You know the right answer?
An imaginary element (x) on mars is composed of three isotopes, 10.68% of isotope x-95 with a mass o...
Questions





Mathematics, 08.01.2021 03:40

English, 08.01.2021 03:40

Mathematics, 08.01.2021 03:40

Mathematics, 08.01.2021 03:40

Mathematics, 08.01.2021 03:40

History, 08.01.2021 03:40

Mathematics, 08.01.2021 03:40




Law, 08.01.2021 03:40

Mathematics, 08.01.2021 03:40


Mathematics, 08.01.2021 03:40


Mathematics, 08.01.2021 03:40

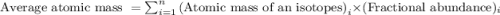 .....(1)
.....(1)![\text{Average atomic mass of X}=[(95\times 0.1068)+(96\times 0.1690)+(97\times 0.7242)]](/tpl/images/0229/1284/93747.png)