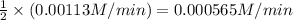Chemistry, 12.09.2019 20:30 owlgirl554
The rate of decomposition of n2o5 in ccl4 at 317 k has been studied by monitoring the concentration of n2o5 in the solution. 2 n2o5(g) → 4 no2(g) + o2(g) initially the concentration of n2o5 is 2.36 m. at 177 minutes, the concentration of n2o5 is reduced to 2.16 m. calculate the average rate of this reaction in m/min.

Answers: 3


Another question on Chemistry


Chemistry, 21.06.2019 22:30
The diagram shows the structures of horse and cat forelimbs. what does the diagram suggest about the evolutionary relationship between these two mammals? a. they have homologous structures, indicating a common ancestor. b. they have analogous structures, indicating a common ancestor. c. they have homologous structures, indicating that they do not have a common ancestor. d. they have analogous structures, indicating that they do not have a common ancestor.
Answers: 2

Chemistry, 22.06.2019 08:00
Joe shines white light into a bowl half full of water at an angle of incident of 27.5°. calculate the angle of refraction in the water given the indices of refraction for air and water are 1.00 and 1.36, respectively.
Answers: 2

You know the right answer?
The rate of decomposition of n2o5 in ccl4 at 317 k has been studied by monitoring the concentration...
Questions


Chemistry, 23.01.2022 04:20




Chemistry, 23.01.2022 04:20





English, 23.01.2022 04:20


English, 23.01.2022 04:20

English, 23.01.2022 04:20






![-\frac{1}{2}\frac{[N_{2}O_{5}]}{\Delta t}=\frac{1}{4}\frac{\Delta [NO_{2}]}{\Delta t}=\frac{\Delta [O_{2}]}{\Delta t}](/tpl/images/0229/1014/2f810.png)
![-\frac{1}{2}\frac{[N_{2}O_{5}]}{\Delta t}](/tpl/images/0229/1014/bf936.png) represents average rate of disappearance of
represents average rate of disappearance of 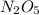 ,
, ![\frac{1}{4}\frac{[NO_{2}]}{\Delta t}](/tpl/images/0229/1014/70f91.png) represents average rate of appearance of
represents average rate of appearance of 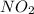 and
and ![\frac{[O_{2}]}{\Delta t}](/tpl/images/0229/1014/eef79.png) represents average rate of appearance of
represents average rate of appearance of 
![-\frac{[N_{2}O_{5}]}{\Delta t}](/tpl/images/0229/1014/beb02.png) =
= 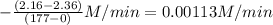
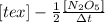 " alt="-\frac{1}{2}\frac{[N_{2}O_{5}]}{\Delta t}" />" /> =
" alt="-\frac{1}{2}\frac{[N_{2}O_{5}]}{\Delta t}" />" /> =