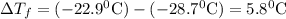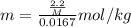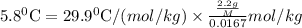Asolution was prepared by dissolving 2.2 g of an unknown solute in 16.7 g of ccl4. a thermal analysis was performed for this solution and it was found that its initial freezing point was – 28.7°c. a reliable source in the bibliography states that for ccl4, t°f = – 22.9°c, and its freezing point lowering constant is kf = 29.9°c/m. calculate the molar mass of the unknown solute.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:00
What is the empirical formula of vanadium 1 oxide given that 20.38 grams of vandium combines with oxygen to form 23.58 grams of the oxide
Answers: 1

Chemistry, 22.06.2019 07:00
What is the main purpose of patent attorneys? defend the company against legal claims manage financial investments invent new products protect rights to new products and processes
Answers: 1


Chemistry, 23.06.2019 00:30
What is bromine+calcium iodide--> calcium bromide +iodine balanced
Answers: 1
You know the right answer?
Asolution was prepared by dissolving 2.2 g of an unknown solute in 16.7 g of ccl4. a thermal analysi...
Questions


English, 22.01.2020 01:31

English, 22.01.2020 01:31


English, 22.01.2020 01:31

Mathematics, 22.01.2020 01:31




Mathematics, 22.01.2020 01:31



Mathematics, 22.01.2020 01:31

Mathematics, 22.01.2020 01:31

History, 22.01.2020 01:31



Mathematics, 22.01.2020 01:31


History, 22.01.2020 01:31

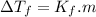
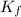 is cryogenoscopic constant of solvent.
is cryogenoscopic constant of solvent.