
Which of the following choices has the compounds correctly arranged in order of increasing solubility in water? (least soluble to most soluble) which of the following choices has the compounds correctly arranged in order of increasing solubility in water? (least soluble to most soluble) lif < nano3 < chcl3 ch3oh < ch4 < lif ch4 < nano3 < chcl3 ccl4 < chcl3 < nano3 ch3oh < cl4 < chcl3

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:10
Seawater contains approximately 3.5%nacl by mass and has a density of 1.02 g/ml. what volume of seawater contains 7.5 g of sodium?
Answers: 2

Chemistry, 22.06.2019 10:30
Consider the following reactions. (note: (s) = solid, (l) = liquid, and (g) = gas.) mg(s) + ½o2(g) → mgo(s) + 146 kcal/mole h2(g) + ½o2(g) → h2o(g), δh = -57.82 kcal/mole what type of reaction is represented by the previous two examples?
Answers: 3

Chemistry, 22.06.2019 22:30
Calculate the concentration of all species in a 0.165 m solution of h2co3.
Answers: 1

Chemistry, 23.06.2019 04:31
What is the amount of energy for a photon that has a 125 cm wavelength
Answers: 2
You know the right answer?
Which of the following choices has the compounds correctly arranged in order of increasing solubilit...
Questions


Geography, 28.09.2019 02:30


English, 28.09.2019 02:30

Mathematics, 28.09.2019 02:30


Chemistry, 28.09.2019 02:30





Computers and Technology, 28.09.2019 02:30







Mathematics, 28.09.2019 02:30

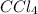 is a non-polar compound. Hence, it is insoluble in water.
is a non-polar compound. Hence, it is insoluble in water. 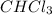 is a polar compound due to difference in electronegativity of chlorine and carbon atom there will be development of partial charges. Hence, there will be dipole-dipole forces existing between them.
is a polar compound due to difference in electronegativity of chlorine and carbon atom there will be development of partial charges. Hence, there will be dipole-dipole forces existing between them. 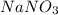 is an ionic compound and it will readily dissociate into ions when dissolved in water. Also, there will be ion-dipole interactions between sodium and nitrate ions.
is an ionic compound and it will readily dissociate into ions when dissolved in water. Also, there will be ion-dipole interactions between sodium and nitrate ions. 

