
Chemistry, 12.09.2019 21:30 Benjamincompton07
Atechnician tares a 100.0 ml volumetric flask at 150.00 g. after adding sodium chloride to the flask it then weighs 158.84 g. assuming an error of 0.2 ml in the volumetric volume and 0.005 g in the weight, calculate the molar concentration of sodium chloride and its associated standard deviation.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Acompound has a molar mass of 92.02 grams/mole, and its percent composition is 30.4% nitrogen (n) and 69.6% oxygen (o). what is its molecular formula? a. n2o4 b. no2 c. n2o d. n4o2
Answers: 1

Chemistry, 22.06.2019 14:30
Select the word from the list that best fits the definition the nuclear family into which a person is born or adopted.
Answers: 2

Chemistry, 22.06.2019 17:30
Upon decomposition, one sample of magnesium fluoride produced 1.65 kg of magnesium and 2.56 kg of fluorine. a second sample produced 1.32 kg of magnesium. part a how much fluorine (in grams) did the second sample produce?
Answers: 2

Chemistry, 22.06.2019 20:00
How are the terms group and period used on the periodic table
Answers: 1
You know the right answer?
Atechnician tares a 100.0 ml volumetric flask at 150.00 g. after adding sodium chloride to the flask...
Questions

Mathematics, 21.05.2021 20:00


Computers and Technology, 21.05.2021 20:00

Mathematics, 21.05.2021 20:00


Chemistry, 21.05.2021 20:00

Mathematics, 21.05.2021 20:00




English, 21.05.2021 20:00

Mathematics, 21.05.2021 20:00


Biology, 21.05.2021 20:00

Mathematics, 21.05.2021 20:00

Mathematics, 21.05.2021 20:00

Mathematics, 21.05.2021 20:00

Chemistry, 21.05.2021 20:00


Mathematics, 21.05.2021 20:00

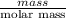
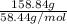
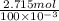 mol/liter
mol/liter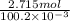 mol/liter
mol/liter 

