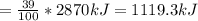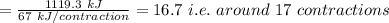Assume that the complete combustion of one mole of glucose to carbon dioxide and water liberates 2870 kj/mol2870 kj/mol ( δ°′=−2870 kj/molδg°′=−2870 kj/mol ). if one contraction cycle in muscle requires 67 kj67 kj , and the energy from the combustion of glucose is converted with an efficiency of 39%39% to contraction, how many contraction cycles could theoretically be fueled by the complete combustion of one mole of glucose? round your answer to the nearest whole number.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
What is i fracture in the crust called when land move up, down or sideways
Answers: 2

Chemistry, 22.06.2019 10:00
A50.0g sample of liquid water at 0.0 c ends up as ice at -20.0 c. how much energy is involved in this change?
Answers: 1

Chemistry, 22.06.2019 14:20
You have a liquid that exhibits diltancy. you want to pour it from a bottle. what should you do to the bottle before pouring
Answers: 1

Chemistry, 22.06.2019 18:10
Measurements that have similar values are: a. usually accurate b. sometimes accurate c. always accurate d. never accurate
Answers: 1
You know the right answer?
Assume that the complete combustion of one mole of glucose to carbon dioxide and water liberates 287...
Questions

Computers and Technology, 05.05.2020 23:31

English, 05.05.2020 23:31

Mathematics, 05.05.2020 23:31

Mathematics, 05.05.2020 23:32


History, 05.05.2020 23:32



Physics, 05.05.2020 23:32


Mathematics, 05.05.2020 23:32



Mathematics, 05.05.2020 23:32




Mathematics, 05.05.2020 23:32