
Chemistry, 13.09.2019 02:30 thereal6ix9ine69
The following five beakers, each containing a solution of sodium chloride (nacl, also known as table salt), were found on a lab shelf:
beaker contents
1) 200. ml of 1.50 m nacl solution
2) 100. ml of 3.00 m nacl solution
3) 150. ml of solution containing 19.5 g of nacl
4) 100. ml of solution containing 19.5 g of nacl
5) 300. ml of solution containing 0.450 mol nacl
arrange the solutions in order of decreasing concentration.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:40
Write the formula for the following chemicals. 7. e. trinitrogen tetraoxide a calcium phosphate f. magnesium acetate b. potassium sulfide g nickel(iii) cyanide c carbon dioxide h. silver sulfate d. cobalt(ii) chloride
Answers: 1

Chemistry, 22.06.2019 09:40
In the lab, ammonia was mixed with water to form ammonium hydroxide. what is/are the reactant(s)? o water and ammonia o ammonia o ammonium hydroxide need
Answers: 2


You know the right answer?
The following five beakers, each containing a solution of sodium chloride (nacl, also known as table...
Questions

Physics, 27.01.2021 02:40

History, 27.01.2021 02:40


Mathematics, 27.01.2021 02:40



Biology, 27.01.2021 02:40


Mathematics, 27.01.2021 02:40



Mathematics, 27.01.2021 02:40

Mathematics, 27.01.2021 02:40

History, 27.01.2021 02:40

Mathematics, 27.01.2021 02:40



Mathematics, 27.01.2021 02:40

Mathematics, 27.01.2021 02:40

Mathematics, 27.01.2021 02:40

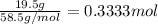
![[NaCl]=\frac{0.3333 mol}{0.150 L}=2.222 M](/tpl/images/0229/5344/75f3b.png)
![[NaCl]=\frac{0.3333 mol}{0.100 L}=3.333 M](/tpl/images/0229/5344/111d7.png)
![[NaCl]=\frac{0.450 mol}{0.300 L}=1.50 M](/tpl/images/0229/5344/58018.png)


