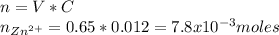Chemistry, 13.09.2019 04:20 ashtor1943
If you had a 0.650 l solution containing 0.0120 m of zn2+(aq), and you wished to add enough 1.34 m naoh(aq) to precipitate all of the metal, what is the minimum amount of the naoh(aq) solution you would need to add? assume that the naoh(aq) solution is the only source of oh−(aq) for the precipitation.

Answers: 3


Another question on Chemistry


Chemistry, 21.06.2019 22:30
200. ml of 3.00 m nacl solution is diluted to a final volume of 500. ml. what is the molarity of the final solution?
Answers: 2


Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 2
You know the right answer?
If you had a 0.650 l solution containing 0.0120 m of zn2+(aq), and you wished to add enough 1.34 m n...
Questions


Mathematics, 04.07.2019 14:00

Social Studies, 04.07.2019 14:00

Mathematics, 04.07.2019 14:00



History, 04.07.2019 14:00


Physics, 04.07.2019 14:00

Mathematics, 04.07.2019 14:00

History, 04.07.2019 14:00

Mathematics, 04.07.2019 14:00

Physics, 04.07.2019 14:00


Chemistry, 04.07.2019 14:00

Biology, 04.07.2019 14:00




Social Studies, 04.07.2019 14:00