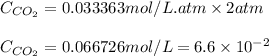Chemistry, 13.09.2019 17:30 NotYourStudent
Suppose the gas above the soda in a bottle of soft drink
ispure co2 at a pressure of 2atm. calculate [co2] at 25
degreec.
henry's law at 25c of co2 is 0.033363 (mol/l*atm).

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Describe the particles of all three phases of matter in the root beer float. (how are the particles arranged and moving? )
Answers: 3

Chemistry, 22.06.2019 10:50
Someone offer some answers to this, i will give 98 coins and mark as brainliest! i will put the rest of the lab down in the comments,solutions pre-lab questions: in this lab, you will make fruit drinks with powdered drink mix. complete the pre-lab questions to get the values you need for your drink solutions. calculate the molar mass of powered fruit drink mix, made from sucrose (c12h22o11).using stoichiometry, determine the mass of powdered drink mix needed to make a 1.0 m solution of 100 ml. (hint: use molarity = to find the moles of drink mix, then convert moles to grams using a mole conversion.)what mass of powdered drink mix is needed to make a 0.5 m solution of 100 ml?
Answers: 1

Chemistry, 22.06.2019 13:30
Ants live on acacia trees in south america. the ants feed on sugars secreted by the trees. the trees provide room for the ants to live. the ants sting any other insect or animal that comes to eat the trees. what type of relationship is this?
Answers: 1

You know the right answer?
Suppose the gas above the soda in a bottle of soft drink
ispure co2 at a pressure of 2atm. cal...
ispure co2 at a pressure of 2atm. cal...
Questions

Mathematics, 26.10.2020 01:00



Mathematics, 26.10.2020 01:00

Mathematics, 26.10.2020 01:00


Mathematics, 26.10.2020 01:00



Mathematics, 26.10.2020 01:00

Mathematics, 26.10.2020 01:00

Computers and Technology, 26.10.2020 01:00

Spanish, 26.10.2020 01:00





History, 26.10.2020 01:00


Mathematics, 26.10.2020 01:00

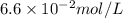
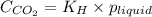
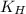 = Henry's constant =
= Henry's constant = 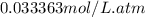
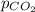 = partial pressure of gas in a bottle of soft drink = 2 atm
= partial pressure of gas in a bottle of soft drink = 2 atm