
Chemistry, 13.09.2019 17:30 michaelbromley9759
Hydrogen reacts with sodium to produce solid sodium hydride. a reaction mixture contains 6.75 g na and 3.03 g hydrogen. which reactant is limiting?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:00
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al
Answers: 1

Chemistry, 22.06.2019 03:00
Schrodinger and heisenberg developed an alternate theory about atomic nature that contradicted some of bohr's model of the atom. how do changes resulting from new technology and evidence affect the reputation of the atomic theory?
Answers: 1

Chemistry, 22.06.2019 04:40
Silver tarnishes as silver metal reacts with hydrogen sulfide, h2s, in the air. in this reaction, dark silver sulfide, au2s, covers the surface of silver. when silver is polished, this coating of silver sulfide can be removed from the surface. this makes the silver shiny again. enter the coefficients that balance the tarnishing reaction equation. (type 1 for no coefficient.)
Answers: 2

Chemistry, 22.06.2019 14:30
How can carbon move from "land" to bodies of water? describe the way human impact has lead to increased levels of co2 in the atmosphere.
Answers: 2
You know the right answer?
Hydrogen reacts with sodium to produce solid sodium hydride. a reaction mixture contains 6.75 g na a...
Questions

Mathematics, 08.11.2020 01:00


English, 08.11.2020 01:00

Mathematics, 08.11.2020 01:00

Mathematics, 08.11.2020 01:00



History, 08.11.2020 01:00

English, 08.11.2020 01:00



Mathematics, 08.11.2020 01:00


Mathematics, 08.11.2020 01:00





Mathematics, 08.11.2020 01:00

Mathematics, 08.11.2020 01:00

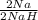 = 0.294mol NaH
= 0.294mol NaH


