
Chemistry, 13.09.2019 20:30 hotrahul8702
10 kg of saturated solution of a highly soluble component a at 80°c is cooled to 30°c calculate the amount of an-hydrous crystals are coming out of the solution solubility of a at 80*c is 0.8 kg of a 1 kg of water and at 30°c is 0.3 kg of a 1 kg of water a) 2.73 kg b) 5.73 kg c) 4.73 kg d) 3.73 kg

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:50
Which statement is a reason to support population regulation? a) it is unethical for us to control birth control rates b) humans have the freedom to produce as many children as desired c) the gap between the rich and poor has been narrowing since 1960 d) billions more people on the earth will intensify many environmental and social problems
Answers: 1

Chemistry, 22.06.2019 00:30
This is a characteristic of the elements in the periodic table that shows a pattern. it may increase or decrease across or down the table.
Answers: 1

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 22.06.2019 11:00
Freezing and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1
You know the right answer?
10 kg of saturated solution of a highly soluble component a at 80°c is cooled to 30°c calculate the...
Questions

Chemistry, 13.10.2019 10:50


History, 13.10.2019 10:50





Physics, 13.10.2019 10:50

Mathematics, 13.10.2019 10:50


Mathematics, 13.10.2019 10:50

Mathematics, 13.10.2019 10:50

Physics, 13.10.2019 10:50

Chemistry, 13.10.2019 10:50



History, 13.10.2019 10:50


Mathematics, 13.10.2019 10:50

English, 13.10.2019 10:50

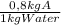 = 8 kg of A
= 8 kg of A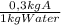 = 3 kg of A
= 3 kg of A

