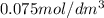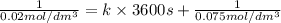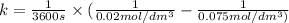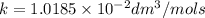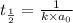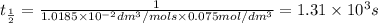Chemistry, 13.09.2019 21:20 haleybug100300
Asecond order reaction of the type a+b> p was carried out in a solution that was initially .075 mol dm-3 in a and .03 mol dm-3 in b. after 1 hour, the concentration of a had fallen to .02 mol dm-3. a. calculate the rate constant. b. what is the half life of the reactant? answers: a. 16.2 dm3mol-hr-, 4.5e-3 dm3mol-s- b. 5.1e3s, 2.1e3 s

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
50 pts plz what is the physical state of matter of baking soda.
Answers: 1

Chemistry, 22.06.2019 03:00
Which step in naming unsaturated hydrocarbons is used for alkenes but not alkynes
Answers: 2

Chemistry, 22.06.2019 04:20
Which formula can be used to calculate the molar mass of ammonia (nh3)? molar mass of n + molar mass of h 3 × molar mass of n + molar mass of h molar mass of n + 3 × molar mass of h 3 × molar mass of n + 3 × molar mass of h
Answers: 1

Chemistry, 22.06.2019 17:30
Observation and experimentation have led many scientists to accept a theory about the origin of the universe. this theory is called the big bang theory. scientific evidence collected and observed by scientists around the world suggests that the universe is ever expanding from a hot and dense initial state. what makes this a scientific theory? (2 points)
Answers: 2
You know the right answer?
Asecond order reaction of the type a+b> p was carried out in a solution that was initially .075 m...
Questions




English, 11.05.2021 16:20

Mathematics, 11.05.2021 16:20

Social Studies, 11.05.2021 16:20




Computers and Technology, 11.05.2021 16:20


Mathematics, 11.05.2021 16:20

Mathematics, 11.05.2021 16:20


Mathematics, 11.05.2021 16:20





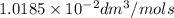 .
. is the half life of the reactant.
is the half life of the reactant.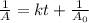
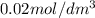
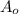 = Initial concentration =
= Initial concentration =