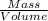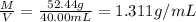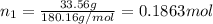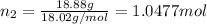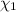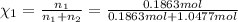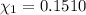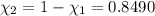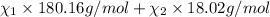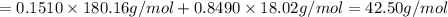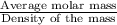Chemistry, 13.09.2019 21:30 sindy35111
33.56 g of fructose (c6h,206) and 18.88 g of water are mixed to obtain a 40.00 ml solution a. what is this solution's density? b. what is the mole fraction of fructose in this solution? c. what is the solution's average molar mass? d. what is the specific molar volume of the solution?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:30
For each of the following compounds, decide whether the compound's solubility in aqueous solution changes with ph. if the solubility does change, pick the ph at which you'd expect the highest solubility. you'll find ksp data in the aleks data tab. compounds does solubility change with ph
Answers: 3

Chemistry, 22.06.2019 16:50
Which element is least likely to undergo a chemical reaction
Answers: 3

Chemistry, 23.06.2019 02:00
Which of the following substances is the most soluble in water? a. sodium chloride b. methane c. bromine d. carbon
Answers: 1

Chemistry, 23.06.2019 08:00
Match the vocabulary terms to their definitions. 1 . a long, chain-like set of molecules made up of repeating units joined end to end polymer 2 . a hard, brittle, heat- and corrosion-resistant material made by subjecting a nonmetallic mineral mixture to intense heat ceramic 3 . a plastic with low elongations that cannot be recycled thermoset 4 . a carbon fiber embedded in a polymer resin matrix thermoplastic 5 . a plastic with high elongations that can be recycled crystal 6 . a solid form resulting from the arrangement of atoms, ions, or molecules in definite geometric patterns composite
Answers: 1
You know the right answer?
33.56 g of fructose (c6h,206) and 18.88 g of water are mixed to obtain a 40.00 ml solution a. what i...
Questions

History, 25.09.2020 03:01




English, 25.09.2020 03:01


History, 25.09.2020 03:01

Mathematics, 25.09.2020 03:01



Mathematics, 25.09.2020 03:01

Engineering, 25.09.2020 03:01




Social Studies, 25.09.2020 03:01


Mathematics, 25.09.2020 03:01

English, 25.09.2020 03:01