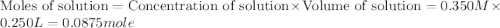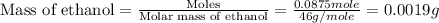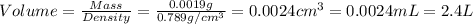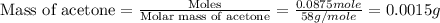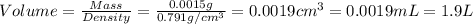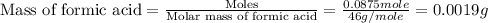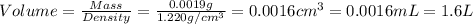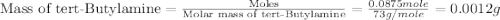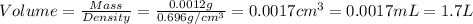Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 11:50
Acompound has a molecular weight of 12.124 atomic mass units and the empirical formula c3h40. what is the molecular formula of the compound?
Answers: 3

Chemistry, 22.06.2019 21:00
What is the chemical formula for the compound formed between sodium and flour one
Answers: 1

Chemistry, 22.06.2019 23:00
What is the measured amount of a product obtained from a chemical reaction?
Answers: 1

Chemistry, 23.06.2019 01:00
Which is true concerning the products and reactants of photosynthesis and cellular respiration? a. the products of photosynthesis are sugars and the reactants of cellular respiration are starches. b. the products of photosynthesis are reactants in cellular respiration. c. oxygen is needed for photosynthesis and is given off in cellular respiration.
Answers: 2
You know the right answer?
Given the densities of the following pure liquids, what volume of each is necessary to make 250 ml o...
Questions




Mathematics, 14.05.2021 23:10

Mathematics, 14.05.2021 23:10

Mathematics, 14.05.2021 23:10



Mathematics, 14.05.2021 23:10

English, 14.05.2021 23:10

Social Studies, 14.05.2021 23:10

Mathematics, 14.05.2021 23:10




Mathematics, 14.05.2021 23:10

Physics, 14.05.2021 23:10


Mathematics, 14.05.2021 23:10