
Chemistry, 13.09.2019 22:30 sosick90501
Question: dimethylhydrazine, the
fuel used in the apollo lunar descentmodule, has a molar mass of
60.10 g/mol. it is made up of carbon, hydrogen, and nitrogen atoms.
the combustion of 2.859g of the fuelin excess oxygen yields 4.190g
of carbon dioxideand 3.428g ofwater. what are the simplest and
molecular formulas fordimethylhydrazine?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:40
Achange in the number of neutrons in an atom will change an blank . when the number of protons changes in an atom, a new element will form.
Answers: 2

Chemistry, 22.06.2019 03:00
Which best describes how johannes kepler developed his laws of planetary motion
Answers: 3

Chemistry, 22.06.2019 09:00
Which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 3

Chemistry, 22.06.2019 13:30
Astudent is trying to create a table that compares hypotheses, theories, and laws. hypothesis theory law do scientific researchers formulate it? yes yes yes does it explain why things happen? yes yes no yes yes yes is it used to make predictions? no yes yes which of the following questions would most likely fill the blank in the table? is it an intelligent guess? is it newly formulated? is it based on observations? has it been proved?
Answers: 1
You know the right answer?
Question: dimethylhydrazine, the
fuel used in the apollo lunar descentmodule, has a molar mass...
fuel used in the apollo lunar descentmodule, has a molar mass...
Questions

Mathematics, 22.04.2021 17:40


Mathematics, 22.04.2021 17:40



English, 22.04.2021 17:40

English, 22.04.2021 17:40













Computers and Technology, 22.04.2021 17:40

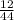 x 4.19g = 1.14g
x 4.19g = 1.14g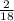 x 3.428g = 0.38g
x 3.428g = 0.38g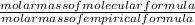 =
= 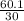 = 2
= 2

