
To find the formula of a compound composed of iron and carbon monoxide, fex(co)y, the compound is burned in pure oxygen, an reaction that proceeds according to the following unbalanced equation.
fex(co)y + o2 --> fe2o3 + co2
if you burn 1.959 g. of fex(co)y and obtain 0.799 g. of fe2o3 and 2.200 g. of co2, what is the empirical formula of fex(co)y?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
If 3.00 g of titanium metal is reacted with 6.00 g of chlorine gas, cl2, to form 7.7 g of titanium (iv) chloride in a combination reaction, what is the percent yield of the product?
Answers: 1

Chemistry, 22.06.2019 03:30
The boiling point of liquids is very high what does it indicate
Answers: 1

Chemistry, 22.06.2019 04:00
Mitosis is a type of cell division that produces cells that are identical to the parent cell. meiosis is a different type of cell division that produces cells that carry have a genetic material of the parent cell. based on the information provided how do the purpose of mitosis and meiosis differ
Answers: 3

Chemistry, 22.06.2019 08:20
What is the formula for the compound dinitrogen pentoxide? a. n4o5 b. n5o4 c. n4o6 d. n5o2 e. n2o5
Answers: 3
You know the right answer?
To find the formula of a compound composed of iron and carbon monoxide, fex(co)y, the compound is bu...
Questions

Mathematics, 10.03.2021 20:10

Mathematics, 10.03.2021 20:10

Physics, 10.03.2021 20:10

English, 10.03.2021 20:10

Geography, 10.03.2021 20:10

Mathematics, 10.03.2021 20:10



Biology, 10.03.2021 20:10


Mathematics, 10.03.2021 20:10

History, 10.03.2021 20:10


Chemistry, 10.03.2021 20:10

English, 10.03.2021 20:10




Mathematics, 10.03.2021 20:10

Mathematics, 10.03.2021 20:10

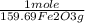 = 5,00×10⁻³ Fe₂O₃ moles
= 5,00×10⁻³ Fe₂O₃ moles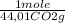 = 5,00×10⁻²CO₂ moles
= 5,00×10⁻²CO₂ moles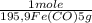 = 1,00×10⁻² Fe(CO)₅ moles
= 1,00×10⁻² Fe(CO)₅ moles

