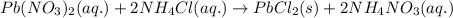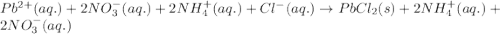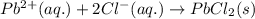Which of the following describes what takes place
whensolutions of pb(no3)2 and
nh4clare...

Chemistry, 13.09.2019 23:10 alessandrotabares
Which of the following describes what takes place
whensolutions of pb(no3)2 and
nh4clare mixed? detail the manner in which the
application of thesolubility rules reveal this answer.
a. pb(no3)2(aq)
+2nh4cl(> nh4no3(aq)
+pbcl2(s)
b. pb2++2cl- --> pbcl2(s)
c. pb2+(aq) +
2no3-(aq)+2nh+4(aq)
+2cl-(> 2nh+4(aq)
+2no3-(aq) + pbcl2(s)
d. nh+4(aq) +
no3-(> 2nh4no32nh4no3(s)
e. no reaction occurs when the solutions aremixed.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Will mark brainliest26. which of these statements are true? (3 points)a. gases are compressibleb. gases fill their containers completelyc. the pressure of a gas is independent of the temperatured. gases have masse. gases exert pressuref. the pressure of a gas is dependent on the volumeg. gas pressure results from the collisions between gas particlesh. gases have a definite volume and shape
Answers: 1

Chemistry, 22.06.2019 12:10
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3

Chemistry, 22.06.2019 17:00
According to the kinetic-molecular theory, what happens to a liquid when it is transferred from one container to another? the volume and the shape stay the same. the volume increases to fill the new container, but the shape stays the same. the volume stays the same, but the shape changes to fit the new container. the volume and the shape change to fill the new container.
Answers: 2

Chemistry, 22.06.2019 20:20
Which symbol can be used to indicate the pressure at which a chemical reaction is carried out? 25°c 2 atm pa
Answers: 2
You know the right answer?
Questions


Mathematics, 31.05.2021 15:30












Mathematics, 31.05.2021 15:30


Biology, 31.05.2021 15:30