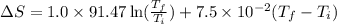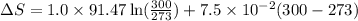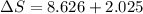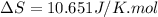in the range 240k to 330k is given

Chemistry, 13.09.2019 23:10 mariaaalopezz
The heat capacity of chloroform (trichloromethane, chcl3)
in the range 240k to 330k is given
bycpm/(jk-1mol-1) = 91.47
+7.5x10-2(t/k). in a particular experiment,
1.0molchcl3 is heated from 273k to 300k. calculate the
changein molar entropy of the sample.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:20
Asolution contains 180 g of glucose (c6h12o6) and 162 g of water. what is the mole fraction of glucose?
Answers: 3

Chemistry, 22.06.2019 22:30
Essay-alternative energy sources research sources of energy that are being developed. write a report of 350-400 words discussing the information you learned concerning the development of various energy sources and the impact that you think they will have on your life. include sources cited at the end of your report using the mla format. follow the rubric guidelines. note that wikipedia is not an appropriate resource for a research paper. worth 99
Answers: 3

Chemistry, 22.06.2019 23:30
Rank substituents in order of their priority when assigning the e or z label to an alkene. i, ch2i , h, ch2ch2cl, f
Answers: 2

Chemistry, 23.06.2019 04:20
The reaction below shows a system in equilibrium. how would a decrease in temperature affect this reaction? a. the rate of formation of the gases would increase. b. the equilibrium of the reaction would shift to the left. c. the equilibrium would shift to cause the gases to sublime into solids. d. the chemicals on the left would quickly form the chemical on the right.
Answers: 1
You know the right answer?
The heat capacity of chloroform (trichloromethane, chcl3)
in the range 240k to 330k is given
in the range 240k to 330k is given
Questions

History, 30.06.2019 13:20


Health, 30.06.2019 13:20


Mathematics, 30.06.2019 13:20

History, 30.06.2019 13:20



Spanish, 30.06.2019 13:20


Mathematics, 30.06.2019 13:20

History, 30.06.2019 13:20

Mathematics, 30.06.2019 13:20


Business, 30.06.2019 13:20


English, 30.06.2019 13:20

Advanced Placement (AP), 30.06.2019 13:20

Mathematics, 30.06.2019 13:20

History, 30.06.2019 13:20

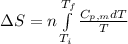
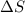 = change in molar entropy
= change in molar entropy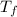 = final temperature = 300 K
= final temperature = 300 K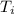 = initial temperature = 273 K
= initial temperature = 273 K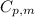 = heat capacity of chloroform =
= heat capacity of chloroform = 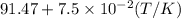
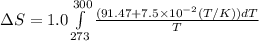
![\Delta S=1.0\times [91.47\ln T+7.5\times 10^{-2}T]^{300}_{273}](/tpl/images/0230/3981/c380e.png)