
Chemistry, 13.09.2019 23:20 michelle230
Calculate the cell potential for a cell operating with the following reaction at 25 degrees celsius, in which [mno4^1-] = .01m, [br^1-] = .01m, [mn^2+] = .15m, and [h^1+] = 1m. the reaction is 2 mno4^1-(aq) + 10 br^1-(aq) + 16 h^1+(aq) --> 2 mn^2+(aq) + 5 br2(l) + 8 h2o(l)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 19:00
Which change to the system wood cause the freely-moving piston to lower?
Answers: 1

Chemistry, 22.06.2019 23:00
Arectangle has a diagonal 20 inches long that forms angles of 60 and 30 with the sides. find the perimeter of the rectangle. for geometry
Answers: 3

Chemistry, 23.06.2019 13:30
Explain the impact that changing the temperature has on a system in a state of dynamic equilibrium. what will happen when the temperature of an exothermic reaction mixture at equilibrium is increased?
Answers: 3

Chemistry, 23.06.2019 14:40
Uuestons niuthe no. of millimoles of hcl required to neutralize 10 ml of 0.2 m na2co3 is(a) 2.0 m mole(b) 4.0 m mole(c) 0.2 m mole(d) 0.4 m mole
Answers: 1
You know the right answer?
Calculate the cell potential for a cell operating with the following reaction at 25 degrees celsius,...
Questions








Computers and Technology, 18.10.2019 07:00



Computers and Technology, 18.10.2019 07:00

Computers and Technology, 18.10.2019 07:00

Computers and Technology, 18.10.2019 07:00

Computers and Technology, 18.10.2019 07:00

Computers and Technology, 18.10.2019 07:00

Computers and Technology, 18.10.2019 07:00

Computers and Technology, 18.10.2019 07:00

Computers and Technology, 18.10.2019 07:00

Computers and Technology, 18.10.2019 07:00

Computers and Technology, 18.10.2019 07:00


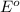 potential will always get reduced and will undergo reduction reaction. Here,
potential will always get reduced and will undergo reduction reaction. Here,  will undergo reduction reaction will get reduced. And, bromine will get oxidized.
will undergo reduction reaction will get reduced. And, bromine will get oxidized. ( × 5)
( × 5)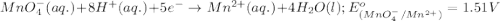 ( × 2)
( × 2)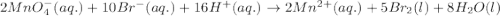
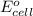 of the reaction, we use the equation:
of the reaction, we use the equation: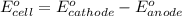
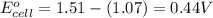
![E_{cell}=E^o_{cell}-\frac{0.059}{n}\log \frac{[Mn^{2+}]^2}{[MnO_4^{-}]^2\times [Br^-]^{10}\times [H^+]^{16}}](/tpl/images/0230/4138/86671.png)
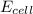 = electrode potential of the cell = ?V
= electrode potential of the cell = ?V![[H^{+}]=1M](/tpl/images/0230/4138/c7b74.png)
![[Mn^{2+}]=0.15M](/tpl/images/0230/4138/f060a.png)
![[MnO_4^{-}]=0.01M](/tpl/images/0230/4138/b97e7.png)
![[Br^{-}]=0.01M](/tpl/images/0230/4138/bb1d7.png)



