Prob.: consider the combustion of butane (c4h10):
2c4h10(g) + 13o2(g) ==> 8co2(g) + 10 h...

Chemistry, 14.09.2019 00:30 zabomoxx5ll
Prob.: consider the combustion of butane (c4h10):
2c4h10(g) + 13o2(g) ==> 8co2(g) + 10 h2o(l)
in a particular reaction, 5.0 moles of c4h10 are reacted
withan excess of o2. calculate the number of moles of co2
formed.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:00
If a plot weight (in g) vs. volume (in ml) for a metal gave the equation y= 13.41x and r^2=0.9981 what is the density of the metal?
Answers: 2

Chemistry, 22.06.2019 04:30
What are the three major branches of natural science? • earth and space science, life science, physical science •earth and space science, physical science, chemistry •physical science, life science, chemistry •life science, chemistry, physics
Answers: 1

Chemistry, 22.06.2019 13:30
What produces wave a)sound b) heats c)transfer of energy d)vibrations
Answers: 2

Chemistry, 22.06.2019 13:50
How does the motion of particles in a gas change as the gas cools
Answers: 2
You know the right answer?
Questions

Mathematics, 20.09.2020 06:01




Mathematics, 20.09.2020 06:01


Engineering, 20.09.2020 06:01





Chemistry, 20.09.2020 06:01


History, 20.09.2020 06:01


Social Studies, 20.09.2020 06:01

History, 20.09.2020 06:01



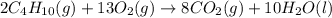
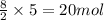 of carbon dioxide.
of carbon dioxide.

