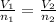Nitrogen oxide is a pollutant commonly found in
smokestackemissions. one way to remove it is t...

Chemistry, 14.09.2019 05:10 angeline2004
Nitrogen oxide is a pollutant commonly found in
smokestackemissions. one way to remove it is to react it with
ammonia.
how many liters of ammonia are required to change 12.8l of
nitrogenoxide to nitrogen gas? assume 100% yield and that all gases
aremeasured at the same temperature and pressure.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 21:00
What is the chemical formula for the compound formed between sodium and flour one
Answers: 1

Chemistry, 22.06.2019 21:30
What is the effect of returning nuclear reactor cooling water back into bodies of water?
Answers: 3

Chemistry, 23.06.2019 00:30
The molecular weight of carbon dioxide, co2, is 44.00 amu, and the molecular weight of nitrous dioxide, no2, is 46.01 amu, so no2 diffuses co2
Answers: 2

You know the right answer?
Questions


Computers and Technology, 06.11.2019 05:31

Mathematics, 06.11.2019 05:31


Social Studies, 06.11.2019 05:31



History, 06.11.2019 05:31

History, 06.11.2019 05:31




Mathematics, 06.11.2019 05:31

Mathematics, 06.11.2019 05:31

World Languages, 06.11.2019 05:31




Mathematics, 06.11.2019 05:31

Social Studies, 06.11.2019 05:31