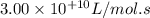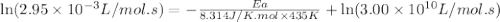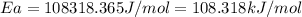Areaction between substances y and z is
by
y2 + z2 > 2yz
the rate constant ob...

Chemistry, 14.09.2019 05:20 johnny2585
Areaction between substances y and z is
by
y2 + z2 > 2yz
the rate constant obeys the arrhenius equation. at 435.
k
the rate constant is k = 2.95 e-03 l/mol-s and a = 3.00 e+10
l/mol-s
what is the activation energy (kj/mol) for
thisreaction?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Will mark brainliest26. which of these statements are true? (3 points)a. gases are compressibleb. gases fill their containers completelyc. the pressure of a gas is independent of the temperatured. gases have masse. gases exert pressuref. the pressure of a gas is dependent on the volumeg. gas pressure results from the collisions between gas particlesh. gases have a definite volume and shape
Answers: 1

Chemistry, 21.06.2019 23:30
What’s the scientific notation for the number 6,840,000,000
Answers: 1


Chemistry, 22.06.2019 03:30
Select the correct answer. when carbon dioxide dissolves in water, it sometimes reacts with water to form carbonic acid as in this balanced equation: co2 + h2o → h2co3. if 495 milliliters of carbon dioxide at 25°c and 101.3 kilopascals reacts with excess water, what is the theoretical yield of carbonic acid? use the periodic table and the ideal gas resource a. 0.889 g b. 1.10g c. 1.27g d. 2.02g what's the answer! quick!
Answers: 1
You know the right answer?
Questions


Mathematics, 07.11.2019 17:31


Computers and Technology, 07.11.2019 17:31

Biology, 07.11.2019 17:31



Mathematics, 07.11.2019 17:31

Health, 07.11.2019 17:31

History, 07.11.2019 17:31

Physics, 07.11.2019 17:31


Mathematics, 07.11.2019 17:31



Arts, 07.11.2019 17:31

English, 07.11.2019 17:31




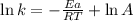 ............(1)
............(1)