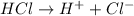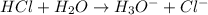Chemistry, 14.09.2019 06:30 theresamarieuehling2
Define the following: bronsted-lowry acid - lewis acid- strong acid - (5 points) problem 6: consider the following acid base reaction hci + h20 → h30+ + cl- a) is this a strong acid? b) clearly label the acid, base, conjugate acid and conjugate base. (5 points)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:50
Which best describes why nh4+ can form an ionic bond with cl-?
Answers: 3

Chemistry, 22.06.2019 18:30
Asample of hydrated tin (ii) chloride (sncl2) has a mass of 4.90 g. when it is dehydrated, it has a mass of 4.10 g. which is the correct chemical formula for the hydrate? sncl2•2h2o sncl2•4h2o sncl2•6h2o
Answers: 2

Chemistry, 22.06.2019 20:30
Consider the following unbalanced equation for the combustion of hexane: αc6h14(g)+βo2(g)→γco2(g)+δh2o(g) part a balance the equation. give your answer as an ordered set of numbers α, β, γ, use the least possible integers for the coefficients. α α , β, γ, δ = nothing request answer part b determine how many moles of o2 are required to react completely with 5.6 moles c6h14. express your answer using two significant figures. n n = nothing mol request answer provide feedback
Answers: 2

Chemistry, 23.06.2019 00:30
What are the advantages of using the metric system? designed as a decimal system making conversions simpler more accurate system of measurement has prefixes that correspond to an amount to use with all base units used by the entire scientific community
Answers: 2
You know the right answer?
Define the following: bronsted-lowry acid - lewis acid- strong acid - (5 points) problem 6: consid...
Questions

English, 08.06.2021 15:30


Geography, 08.06.2021 15:30

Physics, 08.06.2021 15:30

French, 08.06.2021 15:30


Mathematics, 08.06.2021 15:30

Mathematics, 08.06.2021 15:30

Mathematics, 08.06.2021 15:30

English, 08.06.2021 15:30


Mathematics, 08.06.2021 15:30

Mathematics, 08.06.2021 15:30

Social Studies, 08.06.2021 15:30




Mathematics, 08.06.2021 15:30


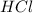 is a strong acid.
is a strong acid. 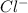 , base =
, base = 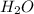 , conjugate acid =
, conjugate acid = 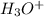
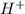 ions.
ions.